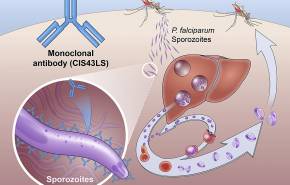
A recent U.S. National Institutes of Health (NIH) clinical trial found for the first time one dose of an antibody drug safely protected healthy, non-pregnant adults from malaria infection during an intense six-month malaria season in Africa.
The antibody was up to 88.2% effective at preventing infection over 24 weeks, demonstrating that a monoclonal antibody can prevent malaria infection in an endemic region.
The NIH says there is an urgent need for new, fast-acting, infrequently dosed interventions that safely protect against malaria infection.
Read More