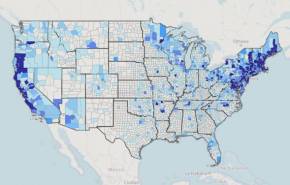
According to an announcement today, a Lyme disease vaccine candidate may soon obtain approval in the U.S. This would be significant since Lyme disease remains a serious health risk to most people.
This vector-borne illness is considered common in the Northern Hemisphere.
Each year, approximately 30,000 cases of Lyme disease are reported to U.S. CDC.
Earlier today, Pfizer Inc. and Valneva SE reported antibody persistence data six months after completing a three-dose or a two-dose vaccination schedule with their Lyme disease vaccine candidate.
Read More