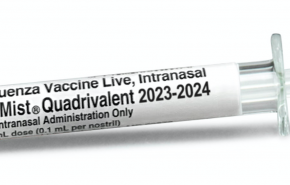
For the first time, an influenza vaccine sprayed into the nose that protects people against the seasonal flu has been accepted for review by the U.S. Food and Drug Administration (FDA).
If approved by the FDA, FLUMIST® QUADRIVALENT will be the first live nasal influenza vaccine available to be self-administered by eligible patients or administered by caregivers.
Read More